Mayadev Lab
- Focused Projects and Grants
- Clinical Trials
- Clinical Trial Advisory Space
- Media
- Patient and Provider Resources
Dr. Mayadev's research involves the development of national clinical trials in cervical cancer, therapeutic early phase clinical trials, and immunotherapy combined with radiation in cervical cancer. Dr. Mayadev is truly a clinician scientist and uses her clinical expertise and experience with patient care to drive innovation. After graduating from radiation oncology residency as chief resident at the University of Washington Medical Center, Seattle, WA, and participating in basic science and clinical research in her training, she launched her laboratory at the University of California. She had an accelerated promotion at UC Davis during her 8 years, and joined the UC San Diego faculty in 2017. In addition to her clinical experience with gynecological patients and brachytherapy, she has been a principal investigator in impactful clinical trials in cervical cancer, and has garnished more than $4.5 Million dollars in grant funding for her collaborative research projects.
She currently oversees the national cervical cancer portfolio as the co-chair of the NCI funded NRG oncology cervical cancer committee. Dr Mayadev is the national principal investigator for GOG 9929 and NRG GY017, phase I NCI funded clinical trials examining the role of immunotherapy with chemoradiation in locally advanced cervical cancer to increase scientific knowledge of the immune system and survivals in patients with node positive disease. Her collaborative R01 grant, " Immunogenomic predictors of outcomes in patients with locally advanced cervical cancer treated with immunotherapy and chemoradiation" using patient samples from the NRG GY017 trial. Her pending R50 grant from the NCI, "NCI Clinical Trial Research Strategy, Harnessing of Equity, and Implementation," allows discoveries for locally advanced cervical cancer in the context of novel treatment options. Furthermore, she was given the NRG Oncology Outstanding Service Award in 2018 for her leadership. She is the co-PI of the largest reported phase III trial in cervical cancer examining immunotherapy with radiation in cervical cancer in 750 patients globally, the CALLA trial. She has extensive experience in brachytherapy, treating more than 7,500 brachytherapy procedures, and developed the "Handbook of Image Guided Brachytherapy", as senior editor to help practitioners with brachytherapy techniques. Dr. Mayadev also partners with the community of outreach and engagement and has research and grants in decreasing the health care disparities in cervical cancer.
Overview
Node positive cervical cancer continues to represent an unmet medical need with poor outcomes with standard radiation and chemotherapy. Dr. Mayadev is the national principal investigator on three NCI funded clinical trials with U10 grant funding. She is also the co-global principal investigator on a randomized phase III trial with AstraZeneca investigating the role of immunotherapy during and after chemoradiation. She is also leading efforts to understand the immune mechanisms of cervical cancer through her lab collaborations and grant funding.
Research Focus
My clinical and translational research is focused on the innovative combination of radiation and immunotherapy for cervical cancer. I designed and was funded from the NCI for two phase I, and one phase II NRG oncology national clinical trials. Through these trials we understand the efficacy safety and toxicity profile of combination immunotherapy and chemoradiation. We also better understand the immune mechanisms responsible for the recognition of cervical cancer by the immune system and the delineation of predictors of response to CTLA-4 and PD-1 blockade.
Funding and Roles
NCI Clinical Trial Research Strategy, Harnessing of Equity, and Implementation Role: PI, Jyoti Mayadev Funding: 887,815.00; NCI, 1 R50 CA282102-01
Publications
Grants
Curebound Discovery Grant from Curebound: 500K; 1-1-25 to 12-31-26. PI: Jyoti Mayadev, co-PI: Sunil Advani, Ramez Eskander, Pandurang Vijayanand
Exploiting tissue factor expression in cervical cancer for precision medicine
Breakthroughs in radiotherapy and immunotherapy have resulted in precision cancer care in cervical cancer, but the chemotherapy used with radiation is non personalized. Our research focuses on a precision oncology strategy by replacing classical chemotherapy with a targeted antibody drug conjugate. We will perform key human tissue testing necessary for the next steps of clinical trial testing of the novel drug with radiation to improve outcomes in women with cervical cancer.
This project allows for innovation in cervical cancer through investigation of cancer targets using human tissue. The Curebound funding is the critical component to perform the studies with our clinical and research integrative team. We are passionate about transforming the lives of cervical cancer patients equipped with this new knowledge from the research lab with the goal to increase cures.
Key Clinical Trials
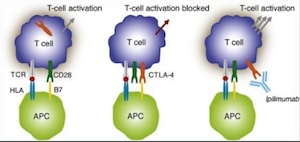 A phase I trial of sequential ipilumumab after chemoradiaiton for cervical cancer, CTEP
A phase I trial of sequential ipilumumab after chemoradiaiton for cervical cancer, CTEP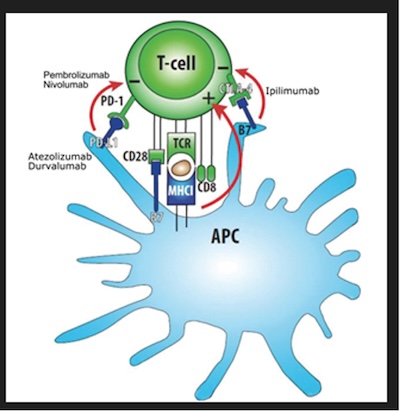
Overview
This series of projects will look at samples from our clinical trials in cervical cancer to investigate the underlying immune responses and predictors for survival after chemoradiation in node positive cervical cancer. The NCI has funded this collaborative project through the NCI R01 mechanism with my collaborator Dr. Dmitriy Zamarin, MD, PhD.
Research Focus
We will determine how the tumor immune microenvironment evolves as a function of differential immunotherapy and CRT sequencing. By using multi-parameter fluorescence microscopy, we will determine how activation of T cells and their interaction with other cells in the tumors change in response to therapy and how these changes predict long term outcomes. We will also investigate T cell receptor (TCR) repertoire sequencing as well as advanced bioinformatics techniques to evaluate how evolution of T cells in tumors and peripheral blood could serve as an indicator of anti-tumor immune response and long-term outcomes. We will establish radiographic and blood biomarkers as predictors of outcomes in high-risk LACC patients by examining blood HPV DNA and post-treatment PET-CT as markers of disease burden pre- and post-therapy. Identification of early biomarkers predictive of outcomes will be critical for risk-stratification of patients with LACC in order to guide patient selection for clinical trials or maintenance therapy, while minimizing the potential clinical toxicities and financial burden in patients at low risk for recurrence.
Funding and Roles
Public Health Relevance
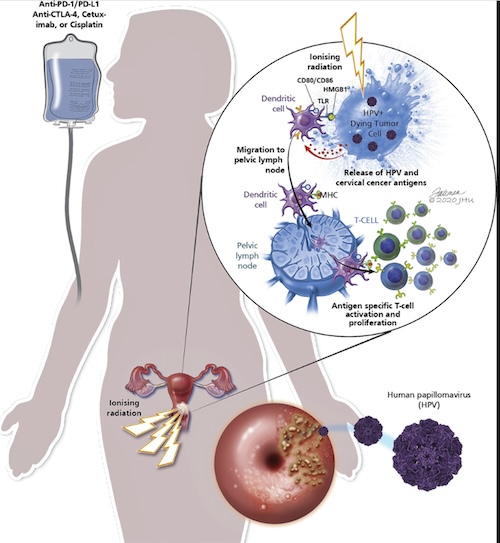 Patients with locally-advanced cervical cancer with lymph node metastases exhibit poor prognosis despite the current standard of care therapy with chemotherapy and radiation. There is thus a critical need to develop new therapeutic strategies for patients with high-risk cervical cancer and to identify biomarkers that could predict which patients are more likely to benefit. The current proposal will take advantage of tumor and blood samples collected from high-risk cervical cancer patients treated on an NCI-funded clinical trial of immunotherapy combined with chemotherapy and radiation to determine optimal sequencing of immunotherapy and radiation and to identify the patients that do or do not benefit from this approach.
Patients with locally-advanced cervical cancer with lymph node metastases exhibit poor prognosis despite the current standard of care therapy with chemotherapy and radiation. There is thus a critical need to develop new therapeutic strategies for patients with high-risk cervical cancer and to identify biomarkers that could predict which patients are more likely to benefit. The current proposal will take advantage of tumor and blood samples collected from high-risk cervical cancer patients treated on an NCI-funded clinical trial of immunotherapy combined with chemotherapy and radiation to determine optimal sequencing of immunotherapy and radiation and to identify the patients that do or do not benefit from this approach.
Overview
Through a series of projects and publications, Dr. Mayadev's lab seeks to understand disparities in brachytherapy use. Currently the lab has funding and a collaboration with San Diego State University and Moores Cancer Center to look at trends within the San Diego region for use and outcomes of brachytherapy.
Publications
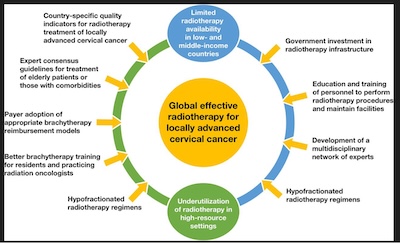 Global challenges of radiotherapy for the treatment of locally advanced cervical cancer.
Global challenges of radiotherapy for the treatment of locally advanced cervical cancer.
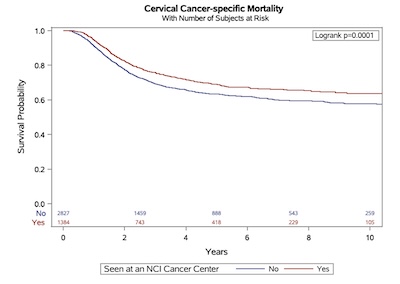 Improved survival in cervical cancer patients receiving care at National Cancer Institute- designated cancer centers.
Improved survival in cervical cancer patients receiving care at National Cancer Institute- designated cancer centers.
Overview
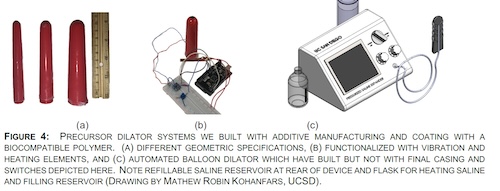
We plan to harness our cervical cancer specific multidisciplinary team of UCSD physicians, bioengineers, and mechanical engineers is addressing this unmet medical need of VS. We will test the hypothesis "radiation induced VS is measureable and patient specific preferences will lead to a personalized engineering device to prevent VS". We intend to provide the input for the engineering personalized vaginal device to mitigate VS for at home use. Our work will eventually lead to the development of a patient specific novel vaginal canal device with a FDA medical device application and an impactful clinical trial.
People
Grants
Grant from the UCSD Academic Senate office, UCSD Engineering GEM grant, MCC Office of Communiy Engagement Pilot grant to study vaginal stenosis, a complication of radiation therapy in gynecologic cancers, pending R21 NCI grant funding (score 13 th percentile).
Available Clinical Trials (open to accrual UCSD)
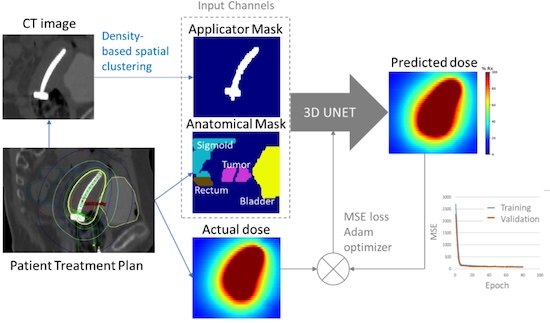
Development and Evaluation of Knowledge-based Cervical Brachytherapy Treatment Planning